Current Research Projects
- The effect of size and shape on plastic deformation of iron and molybdenum nanoparticles on sapphire
- Synthesis and magnetic properties of Au-Fe nano-onions and core-shell nanoparticles
- Porous metal matrix – hydride-carbon nanotubes composites for hydrogen storage
- Diffusion-assisted synthesis and thermal stability of metal nanotubes and nano-channels
Research Highlights
1. Properties of grain boundaries
The grain boundary is a defect of crystalline structure presented in abundance in any solid polycrystalline material. Geometrically, the grain boundary is a plane separating two crystallites of different orientations. Very often the materials break down during service because the atomic bonding at the grain boundaries is weaker than in the crystallites bulk.
The energy of grain boundary is a fundamental parameter describing the work needed to produce 1 m2 of the grain boundary in otherwise perfect crystal (this definition is very similar to the definition of surface energy). Usually, the grain boundaries of high energies also exhibit weaker atomic bonding, higher diffusivity and lower corrosion resistance than their counterparts of low energy. Minimizing the fraction of high-energy grain boundaries in material is one of the ways of improving its properties.
We study the energies of grain boundaries using thermal grooving technique. The dihedral angle formed at the location where the grain boundary emerges at the free surface is related to the energy of a grain boundary and of a free surface.
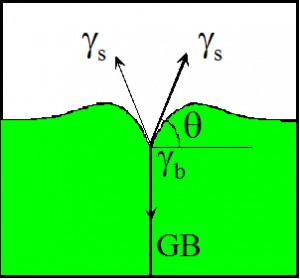
From the dihedral angle q at the root of the groove the energy of the grain boundary, gb, can be determined according to
![]()
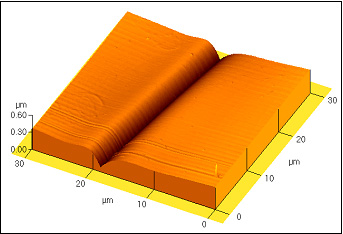
Several examples of the grain boundary grooves in various materials measured by atomic force microscope are given below.
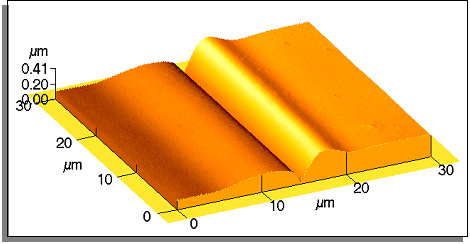
Ni-43 at.% Al (CsCl atomic structure); 1 h at 1400°C.
Pb droplet on Fe; 700°C, 4h
2. Nanoscale mechanical properties
Mechanical properties of small sub-micrometer size samples, as well as mechanical properties of any material when deformed at the nanoscale are very different from the mechanical properties of bulk samples. In metals, the difficulty to nucleate the dislocations in small samples or in small deformed volumes leads to an exceptional increase in strength. We use the nanoindentation instrument (TriboscopeTM of Hysitron) to explore this very interesting field. For instance, we were able to characterize the nanomechanical behavior of individual single crystalline gold nanoparticles and correlate it with the particles size.
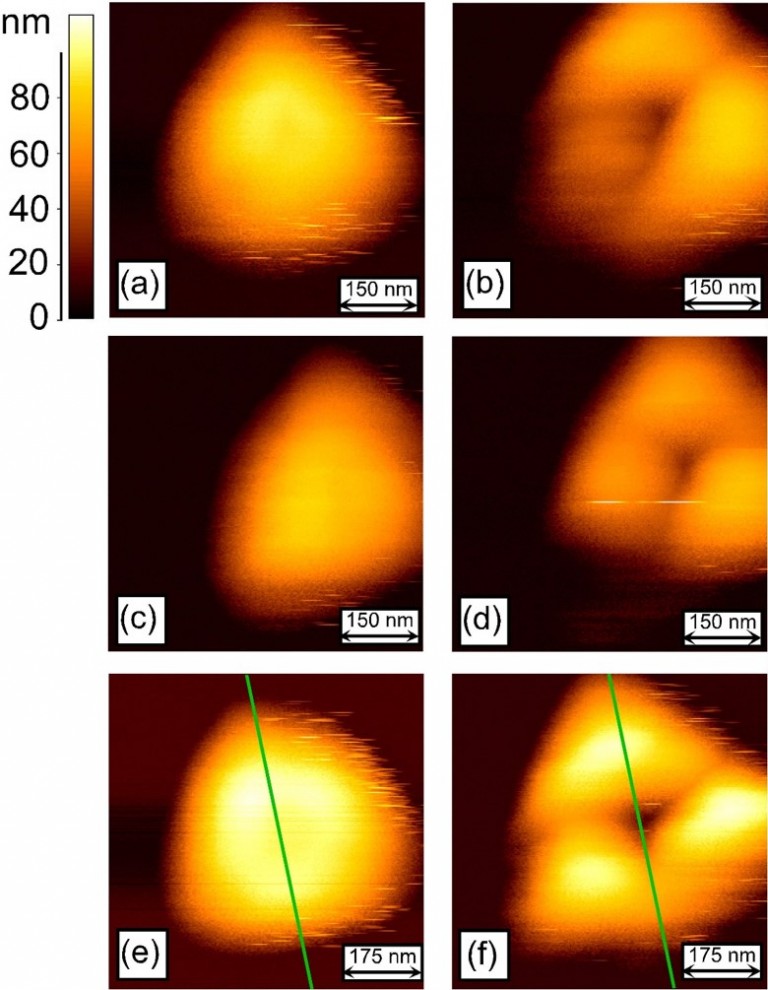
The AFM topography images of gold nanoparticles
on sapphire before (a, c, e) and after (b, d, f)
nanoindentation test with cube corner indenter.
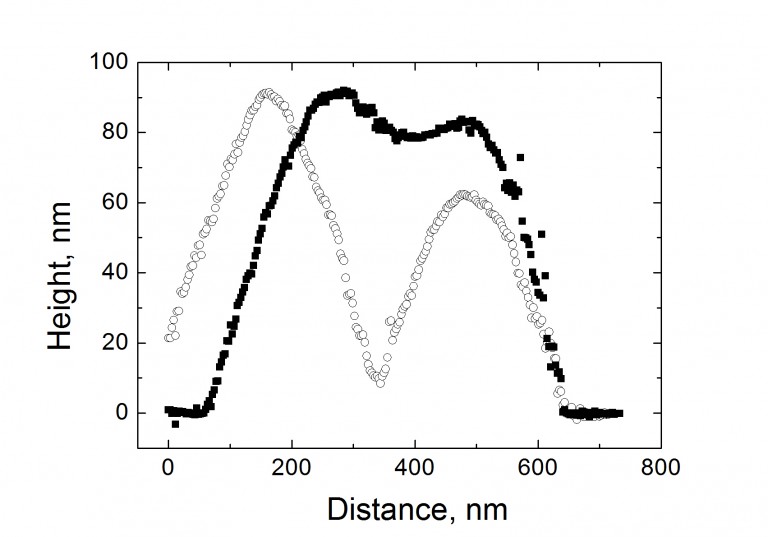
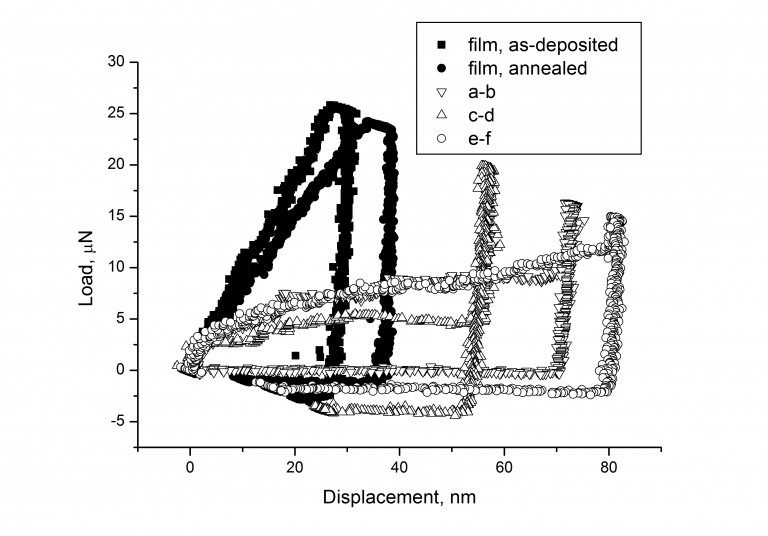
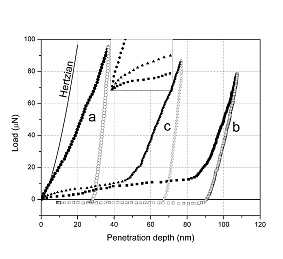
The linear topography profile along the “green line” (see Figure above) demonstrating the
sideward deformation of the particle (a), and the load-displacement curves for the particles,
as compared with the same curves for the gold thin film of comparable thickness (b).
We also try to obtain insight into atomistic mechanisms of plastic deformation in small dimensions by performing molecular dynamic computer simulations of the small samples. These simulations help us to understand the mechanisms of dislocations nucleation. In the movie below the dislocation nucleation events in the gold nanoparticle indented by the cube corner tip is shown. A comparison of the results of atomistic simulations with the experimental data allowed us to draw a conclusion that annihilation of dislocations nucleated beneath the indenter at the lateral surfaces of the nanoparticles determines their strength.
In this example, the particle is shown “from below”, and all internal atoms in the ideal fcc coordination are removed. The atoms with hcp coordination are shown in grey, and they mark the stacking faults associated with nucleating and propagating Shockley partial dislocations.
In the following example, we studied the agglomeration forces between the silver nanoparticles in agglomerates deposited on Si substrate. We were able to characterize the “strong” and “weak” agglomerates by their response to nanoindentation with the sharp “cube corner” indenter. The following topography image is taken from the indented agglomerate of silver nanoparticles. The typical force-displacement curves show that a kind of “plastic flow” at relatively low levels of load is characterizing weak agglomerates.
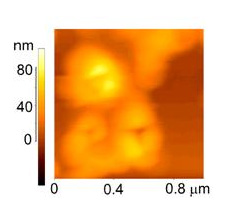
A nanoindenter topography image
of an agglomerate of silver
nanoparticles after indentation.
3. Thermal stability and agglomeration of thin metal films
Solid-state dewetting, or agglomeration of thin films is a process by which a thin continuous film exposes a substrate and transforms into an ensemble of isolated particles. The driving force for dewetting is the reduction of the total surface and interfacial energy of the system, and this process is well-known for metal films on ceramic substrates, especially oxides. The thermal stability of metallic thin films on ceramic substrate is of utmost technological importance. In many cases their adhesion to the substrate may become a “weak link” in the device, leading to its failure when exposed to a sufficient thermal budget. Thus achieving control of their microstructure as well as understanding the dewetting kinetics is a key to preventing device failure caused by film agglomeration. Additionally, such control is also desired when agglomeration is in fact encouraged, as in the case of producing arrays of particles of micron and sub-micron scales, for example for catalytic growth of semiconductor nanowires or for surface enhanced Raman spectroscopy.
We are studying the kinetics of agglomeration of thin Au, Au-Fe and Fe films. Our studies of the microstructure of the films are accompanied by development of kinetic models which allow extracting the surface diffusion coefficients from the kinetic data. Special attention is paid to the role of surface anisotropy (both in terms of surface energy and surface self-diffusion) in films agglomeration.
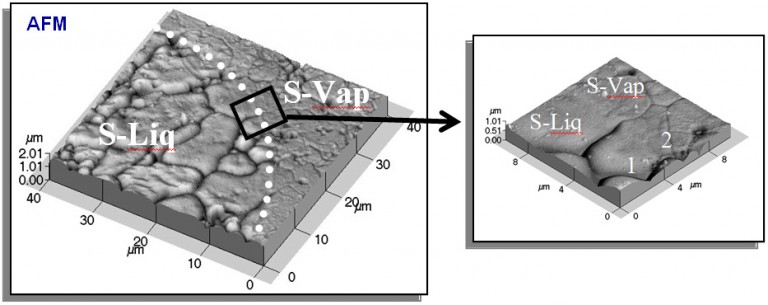
The example below illustrates a spectacular role played by grain boundaries in agglomeration of thin films. In the atomic force microscopy (AFM) images shown below a comparison is made between the single-crystalline Au(Fe) film (9 nm Au, and 12 nm of total thickness) and a polycrystalline Au film (9 nm in thickness), deposited and annealed under identical conditions (7 h at 650°C). The differences of surface morphology between the two films exemplified by the AFM images are immense. In the polycrystalline film the dewetting was complete and fine Au particles cover the substrate. In the single-crystalline film, on the other hand, most of the surface was still covered by the continuous film and only isolated hexagonal holes represent a sign of the onset of the dewetting process. In fact, the Au(Fe) film agglomerated into particles only after annealing for several days at 900 °C.
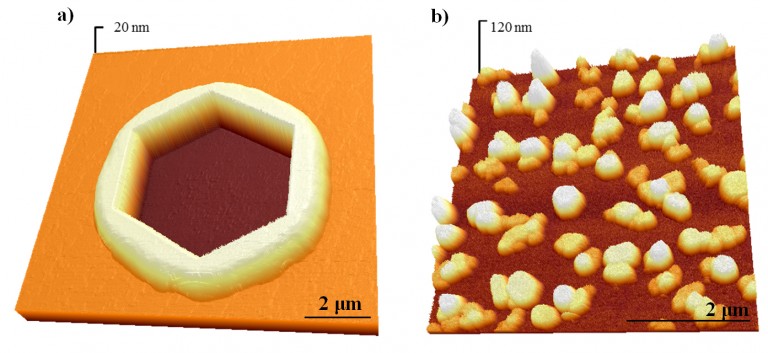
AFM micrographs of (a) Au-Fe single-crystalline bilayer (9 nm-thick Au layer on 3 nm-thick Fe underlayer)
on sapphire and (b) Au polycrystalline layer (9 nm-thick). Both films were deposited under the same
condition and annealed at 650°C for 7 h. The remarkable differences in thermal stability are clearly seen.
4. Hydrogen storage in metal hydrides
Hydrogen is considered as an attractive environmentally friendly alternative to the fossil fuels in the transportation sector. A reliable, light, safe and inexpensive hydrogen storage system is one of the key elements of the future hydrogen economy. For a long time metal hydrides have been considered as a hydrogen storage solution highly competitive with the other storage options (high pressure tanks, liquefaction). However, none of the metal hydrides known today can satisfy the requirements set by automotive industry for the future hydrogen-driven vehicle in terms of the operating temperatures, desorption pressure and kinetics.
We are working on improving the hydrogen storage properties of Mg-based alloys by nanostructuring them using various processing techniques such as equal channel angular pressing (ECAP) and high energy ball milling. The following micrograph demonstrates how ECAP helped to refine the microstructure of Mg-Ni eutectic alloy. The alloy with refined microstructure exhibited higher hydrogen desorption pressures than its as-cast counterpart. Still, there is a long way to go until these alloys can be used as hydrogen storage media in cars. A major breakthrough in this area would bring enormous benefits to the Humankind, and we are working on this!
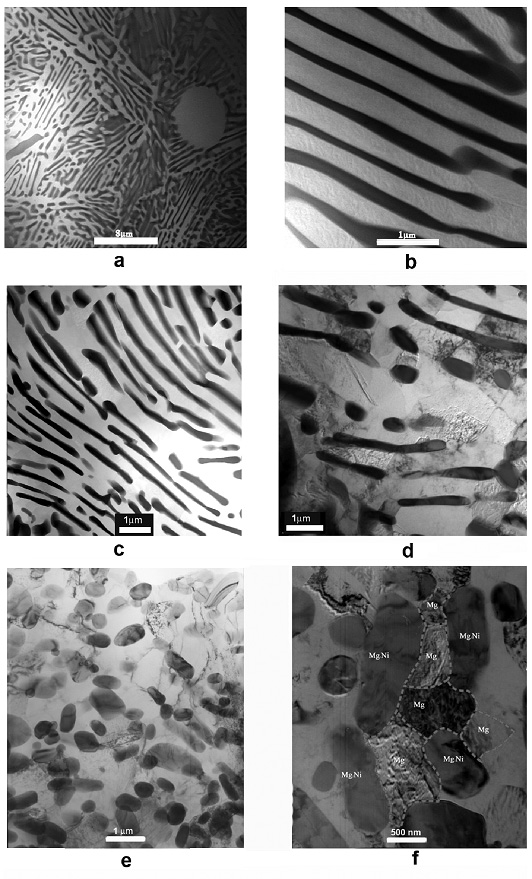
The transmission electron micrographs of Mg-Ni eutectics in the as-cast state (a, b), after 1 ECAP pass (c-d), and after 10 ECAP passes.
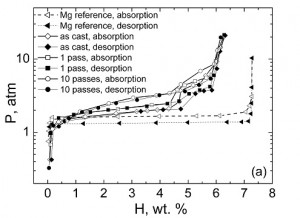
Pressure-composition isotherms at 300 C showing that nanostructuring with ECAP increases hydrogen desorption pressure.
SELECTED PUBLICATIONS
- L.S. Chang, E. Rabkin, B.B. Straumal, B. Baretzky and W. Gust, “Thermodynamic aspects of the grain boundary segregation in Cu(Bi) alloys”,Acta mater.47, 4041-4046, (1999).Full text PDF
- V.M. Skripnyuk, E. Rabkin, Y. Estrin, and R. Lapovok, “The Effect of Ball milling and Equal Channel Angular Pressing on the Hydrogen Absorption/Desorption Properties of Mg-4.95 wt.%Zn-0.71 wt.% Zr (ZK60) Alloy”,Acta mater.52, (2004) 405-414.Full text PDF
- E. Rabkin, D.J. Srolovitz,”Onset of plasticity in gold nanopillar compression”Nanoletters,7(2007) 101-107.Full text PDF
- Y. Amouyal, S.V. Divinski, L. Klinger, E. Rabkin“Grain boundary diffusion and recrystallization in ultrafine grain copper produced by equal channel angular pressing”Acta mater.56(2008) 5640-5652
- E. Rabkin, J.K. Deuschle, B. Baretzky“On the nature of displacement bursts during nanoindentation of ultrathin Ni films on sapphire”Acta mater.58(2010) 1589-1598
- V.M. Skripnyuk, E. Rabkin, L.A. Bendersky, A. Magrez, E. Carreño-Morelli, Y. Estrin“Hydrogen storage properties of as-synthesized and severely deformed magnesium – multiwall carbon nanotubes composite”Int. J. of Hydrogen Energy35(2010) 5471-5478
- D. Mordehai, S.-W. Lee, B. Backes, D.J. Srolovitz, W.D. Nix, E. Rabkin “Size effect in compression of single-crystal gold microparticles” Acta mater. 59 (2011) 5202-5215
- D. Mordehai, E. Rabkin, D.J. Srolovitz “Pseudoelastic deformation during nanoscale adhesive contact formation”Phys. Rev. Lett. 107 (2011) 096101 (1-4)
- V.M. Skripnyuk, E. Rabkin “Mg3Cd: a model alloy for studying the destabilization of magnesium hydride” Int. J. of Hydrogen Energy 37 (2012) 10724-10732
- E. Rabkin “Flattening of a passivated solid surface due to capillarity” Scripta mater. 67 (2012) 625-628
- O. Kovalenko, J.R. Greer, E. Rabkin „Solid state dewetting of thin iron films on sapphire substrates controlled by grain boundary diffusion” Acta mater. 61 (2013) 3148-3156
- E. Rabkin “Metastable porosity in thin polycrystalline films” Scripta mater. 69 (2013) 764-767
- D. Amram, L. Klinger, N. Gazit, H. Gluska, E. Rabkin “Grain boundary grooving in thin films revisited: the role of interface diffusion” Acta mater. 69 (2014) 386-396
- D. Amram, E. Rabkin “Core (Fe) – Shell (Au) nanoparticles obtained from thin Fe/Au bilayers employing surface segregation” ACS Nano 8 (2014) 10687-10693
- S. Baylan, G. Richter, M. Beregovsky, D. Amram, E. Rabkin “The kinetics of hollowing of Ag-Au core-shell nanowhiskers controlled by short-circuit diffusion” Acta mater. 82 (2015) 145-154


