DSC measures the enthalpies associated with transitions and chemical reactions and determines the temperature at which these processes occur.
The instrument allows :
- Determine melting point and enthalpy of fusion
- Crystallization and super-cooling behavior
- Solid-solid transitions and polymorphism
- Glass transition and amorphous materials
- Chemical reactions, decomposition reactions, vulcanization and polymerization
- Calculate enthalpies of reactions
Instrument specifications
- Temperature range: -150oC to 700 °C
- Heating rate: 0.2 to 300 K/min
- Sample: Solid, liquid
- TAWN resolution (FRS/HSS): 0.12/0.2
- TAWN sensitivity (FRS /HSS): 11.9/56
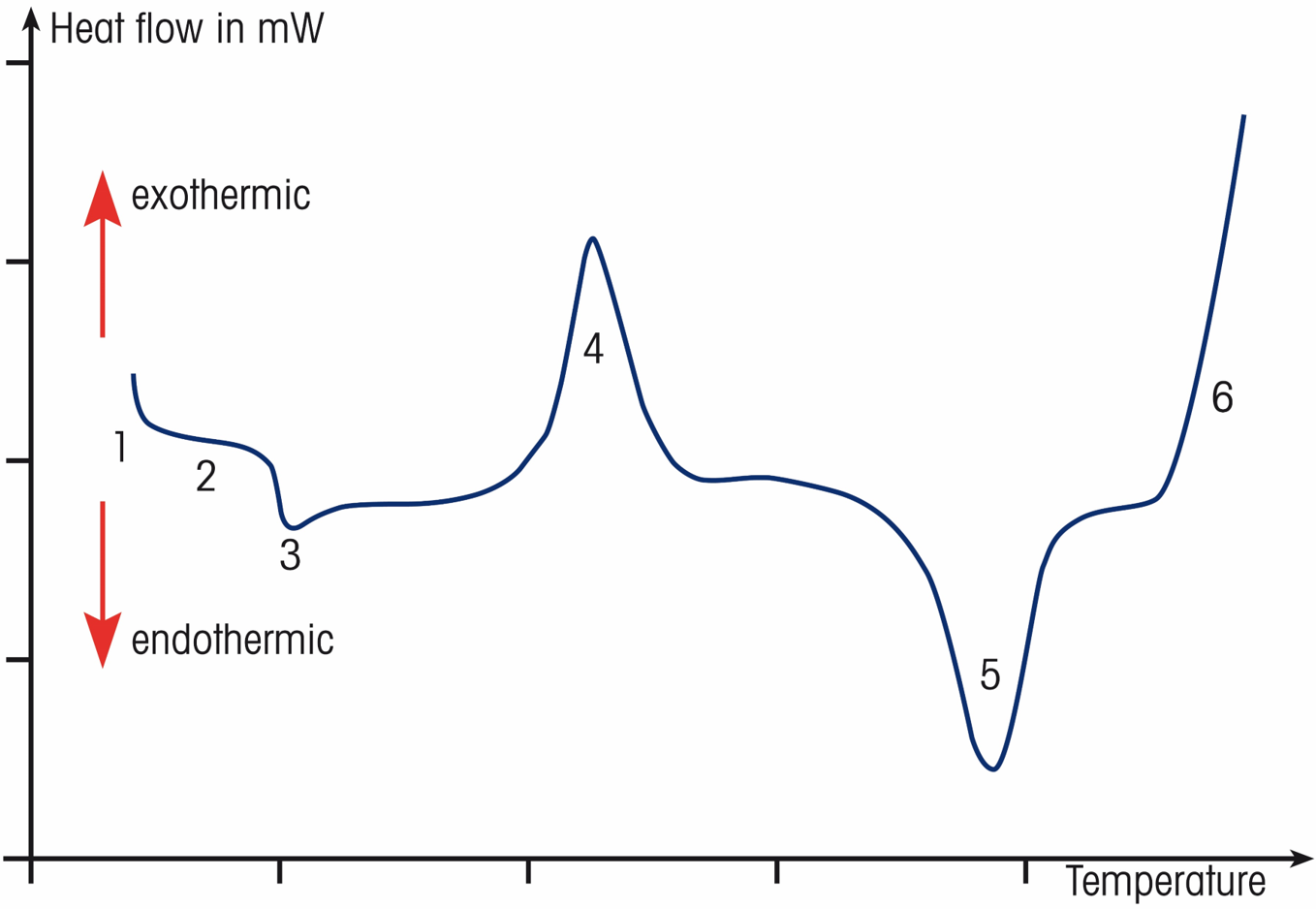
A typical DSC curve of a semi crystalline polymer:
- Initial deflection proportional to the sample’s heat capacity
- DSC curve with no thermal effect (baseline)
- Glass transition of amorphous fraction
- Cold crystallization
- Melting of the crystalline fraction
- Oxidative degradation in air